HSPB8 Protein and the CASA Complex
HSPB8 protein belongs to the family of HSPBs, consisting of ten members. HSPBs recognize misfolded proteins in near-native state, prevent their future misfolding and facilitate refolding by HSPA/HSP70 (Tedesco et al, 2023b).
HSPB8 forms a stable complex, knows as the Chaperone Assisted Selective Autophagy (CASA) complex with chaperone HSPA, cochaperone BAG3 and E3 ubiquitin ligase STUB1. CASA is a highly selective pathway for disposal of misfolding and aggregating proteins, mostly studies in the skeletal muscle and neurons.
Figure 1 - From Tedesco et al., 2023b The chaperone-assisted selective autophagy (CASA) complex. (A) Schematic representation of the CASA complex. BAG3 (green) mediates the assembly of the CASA complex, thereby linking chaperones of the HSPA family (purple) to small heat shock proteins such as HSPB8 (blue). The HSPA-associated E3 ubiquitin ligase STUB1 (orange) interacts with the complex for the ubiquitination of the bound client protein. (B) Engulfment of the CASA complex by LC3-decorated phagophore membrane is facilitated by the autophagic cargo receptor SQSTM1, leading to formation of double-membrane vesicles (called autophagosomes) and final degradation of its content by fusion with the lysosomes
In muscle, CASA maintains muscle integrity by facilitating turnover of structural muscle components damaged by mechanical strain. In neurons, it promotes the degradation of aggregating substrates that cause neurodegenerative diseases.
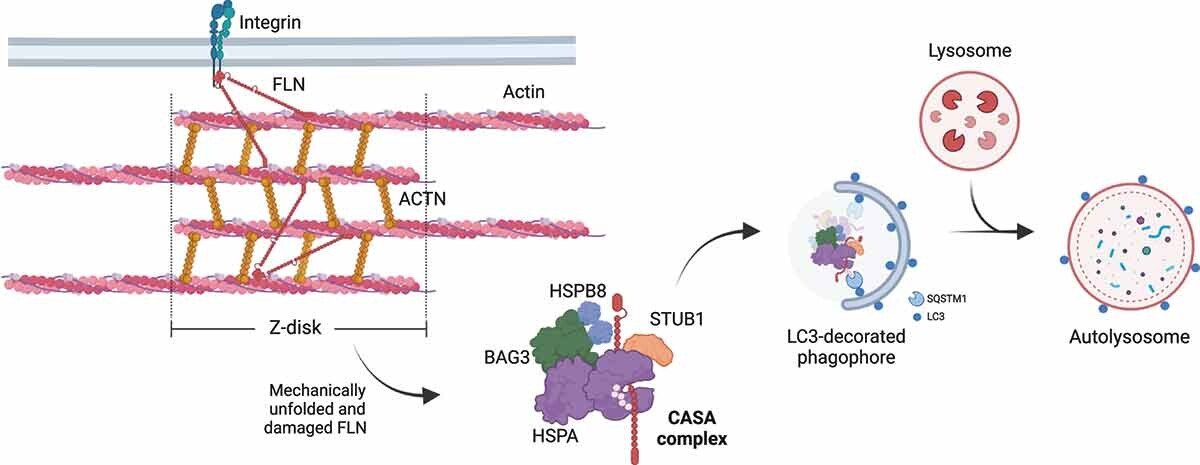
Figure 2-From Tedesco et al., 2023 - CASA-mediated degradation of Z-disk component FLNC. Mechanically unfolded and damaged forms of FLN are recognized and degraded by the CASA pathway. Here, BAG3 closely cooperates with HSPB8 (the “holdase”) and regulates the ATP-dependent chaperone cycle of HSPA, necessary for substrate processing. STUB1-mediated ubiquitination and subsequent recruitment of the autophagic receptor SQSTM1 initiates autophagosome formation, followed by lysosomal fusion for degradation.
Damaged or misfolded proteins are recognized by HSPB8 and HSPA and can be subjected to refolding (driven by HSPA) or ubiquitination (driven by STUB1). Ubiquitinated substrates are degraded via the autophagosome-lysosomal pathway. In case of degradation failure, the misfolded substrates are compartmentalized into aggresomes. The CASA complex is also involved in stress granules maintenance (granulostasis) (Tedesco et al, 2023b).
Mutations in the CASA complex have been linked to several human diseases. Mutations in BAG3 lead to myopathies, neuropathies and cardiomyopathies (reviewed in Tedesco et al, 2023b). Similarly, mutations in HSPB8 lead to neuropathies, and myopathies. However, mutations in STUB1 result in a different phenotype – spinocerebellar ataxia. CASA also is being investigated as a pharmacological target. Several compounds have been found to upregulate HSPB8 expression, including colchicine, doxorubicin and trehalose (Crippa et al, 2016; Chierichetti et al, 2023).